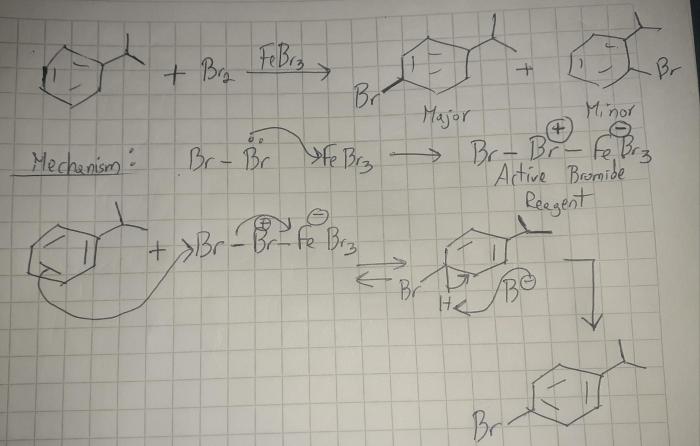
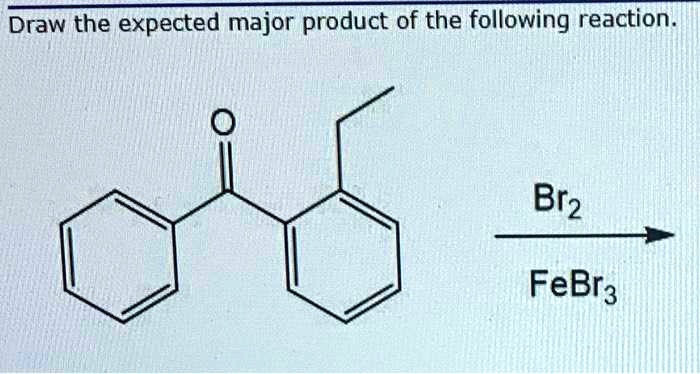
In the realm of organic chemistry, the concept of monobromination reactions emerges as a fundamental tool for regioselectively introducing a bromine atom into an organic molecule. Draw the major monobromination product of this reaction. is a phrase that encapsulates the essence of this transformative process, guiding chemists toward predicting and synthesizing the desired brominated products with precision.
This comprehensive guide delves into the intricacies of monobromination reactions, unraveling the mechanisms, factors, and applications that govern their outcomes. Prepare to embark on an enlightening journey as we explore the depths of this captivating chemical transformation.
Monobromination Reaction Overview
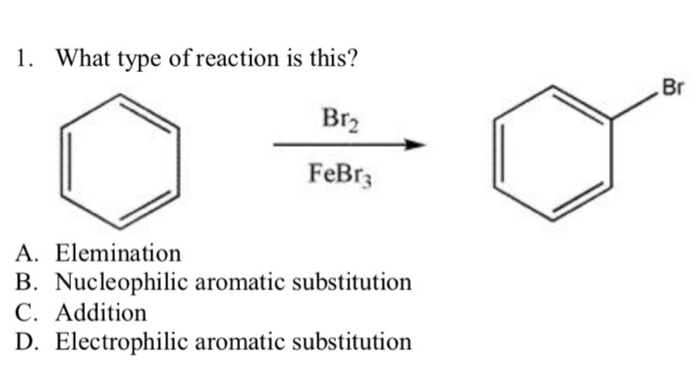
Monobromination reactions introduce a single bromine atom into an organic molecule. These reactions are widely used in organic synthesis to modify the structure and reactivity of organic compounds.
A classic example of a monobromination reaction is the addition of bromine to an alkene, resulting in the formation of a vicinal dibromide:
- CH 2=CH 2+ Br 2→ CH 2Br-CH 2Br
The regioselectivity of monobromination reactions, which determines the position of the bromine atom on the substrate, is influenced by various factors, including steric and electronic effects.
Mechanisms of Monobromination
Monobromination reactions can proceed via two primary mechanisms: the radical mechanism and the electrophilic addition mechanism.
Radical Mechanism
The radical mechanism involves the homolytic cleavage of the bromine-bromine bond, generating two bromine radicals. These radicals then react with the substrate to form a new radical intermediate, which subsequently abstracts a hydrogen atom from the solvent to yield the monobrominated product.
Electrophilic Addition Mechanism
In the electrophilic addition mechanism, bromine acts as an electrophile and adds to the substrate via a concerted process. This mechanism is typically observed with alkenes and alkynes, where the bromine molecule adds across the double or triple bond.
Factors Affecting Regioselectivity
Steric Effects
Steric effects play a crucial role in determining the regioselectivity of monobromination reactions. The presence of bulky substituents near the reaction site can hinder the approach of the bromine molecule, favoring the formation of the product with the bromine atom on the less substituted carbon.
Electronic Effects
Electronic effects also influence the regioselectivity of monobromination reactions. Electron-withdrawing groups, such as carbonyl groups or cyano groups, can stabilize the radical intermediate formed in the radical mechanism, leading to the preferential formation of the product with the bromine atom adjacent to the electron-withdrawing group.
Predicting the Major Monobromination Product, Draw the major monobromination product of this reaction.
Predicting the major monobromination product involves considering both steric and electronic effects:
- Identify the most substituted carbon adjacent to the double or triple bond.
- If the most substituted carbon is adjacent to an electron-withdrawing group, predict the formation of the product with the bromine atom on the adjacent carbon.
- If the most substituted carbon is not adjacent to an electron-withdrawing group, predict the formation of the product with the bromine atom on the less substituted carbon.
Applications of Monobromination
Monobromination reactions have numerous applications in organic synthesis, including:
- The synthesis of vicinal dibromides, which are useful intermediates for the preparation of alkenes and alkynes.
- The selective bromination of alkenes and alkynes to control the regio- and stereochemistry of the products.
- The introduction of bromine atoms into aromatic compounds for the synthesis of substituted aromatic compounds.
FAQs: Draw The Major Monobromination Product Of This Reaction.
What is the significance of regioselectivity in monobromination reactions?
Regioselectivity dictates the specific site of bromine addition within the organic molecule, influencing the product’s structure and properties.
How do steric and electronic effects impact the regioselectivity of monobromination?
Steric effects arise from the physical hindrance of bulky groups, while electronic effects stem from the distribution of electron density within the molecule. Both factors influence the accessibility and reactivity of different carbon atoms toward bromine addition.
Can you provide an example of a synthetic application of monobromination reactions?
Monobromination reactions find widespread use in the synthesis of various organic compounds, including pharmaceuticals, agrochemicals, and fragrances.