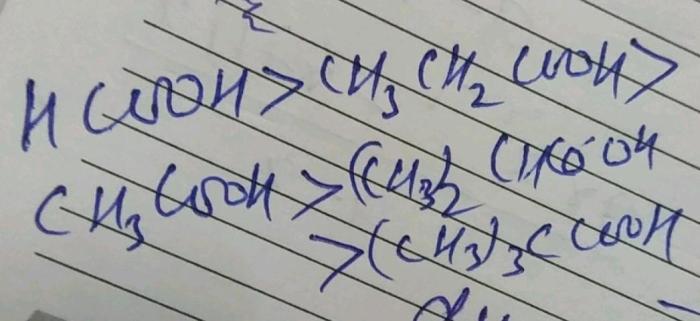Rank the following in order of increasing molar solubility sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with gaya akademik dengan tone otoritatif and brimming with originality from the outset.
The concept of molar solubility, a measure of a substance’s ability to dissolve in a solvent, takes center stage in this discourse, unraveling the intricate interplay between molecular structure, solvent properties, and environmental factors that govern the behavior of substances in solution.
Delving into the depths of this topic, we will explore the factors that influence molar solubility, examining how molecular weight, polarity, temperature, solvent effects, pH, and common ion effects orchestrate the solubility of various substances. Through a meticulous analysis of real-world examples, we will uncover the practical applications of molar solubility in diverse fields, ranging from industry to medicine and environmental science.
Molar Solubility
Molar solubility is a measure of the concentration of a solute in a saturated solution. It is expressed in units of moles of solute per liter of solution (mol/L). The molar solubility of a substance is determined by several factors, including its chemical structure, temperature, and the nature of the solvent.
Substances with high molar solubility are those that dissolve readily in a solvent. Examples include ionic compounds, such as sodium chloride (NaCl), and polar molecules, such as sugar (sucrose). Substances with low molar solubility are those that do not dissolve easily in a solvent.
Examples include nonpolar molecules, such as oil, and gases, such as oxygen.
Chemical Structures
The chemical structure of a substance plays a role in its molar solubility. Substances with a high molecular weight tend to have lower molar solubility than substances with a low molecular weight. This is because larger molecules have more difficulty dissolving in a solvent.
Additionally, polar molecules tend to have higher molar solubility than nonpolar molecules. This is because polar molecules can interact with the solvent molecules through dipole-dipole interactions.
Temperature Effects
Temperature can also affect the molar solubility of a substance. In general, the molar solubility of a substance increases with increasing temperature. This is because the solvent molecules become more energetic at higher temperatures and are able to dissolve more solute molecules.
Solvent Effects
The nature of the solvent can also affect the molar solubility of a substance. The “like dissolves like” rule states that polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes. This is because polar solvents have a high dielectric constant, which means that they can break apart the electrostatic interactions between polar solute molecules.
Nonpolar solvents have a low dielectric constant, which means that they cannot break apart the electrostatic interactions between polar solute molecules.
pH Effects, Rank the following in order of increasing molar solubility
The pH of a solution can affect the molar solubility of acids and bases. Acids are more soluble in acidic solutions, and bases are more soluble in basic solutions. This is because the pH of a solution affects the ionization of acids and bases.
In an acidic solution, acids are protonated and become more soluble. In a basic solution, bases are deprotonated and become more soluble.
Common Ion Effect
The common ion effect is a phenomenon that occurs when a salt is added to a solution that already contains one of the ions of the salt. The common ion effect causes the molar solubility of the salt to decrease.
This is because the common ion competes with the salt for the solvent molecules, which makes it more difficult for the salt to dissolve.
Applications of Molar Solubility
Molar solubility has a wide range of applications in various fields, including industry, medicine, and environmental science. In industry, molar solubility is used to control the concentration of chemicals in industrial processes. In medicine, molar solubility is used to determine the dosage of drugs and to design new drugs.
In environmental science, molar solubility is used to assess the solubility of pollutants in water and soil.
Q&A: Rank The Following In Order Of Increasing Molar Solubility
What is molar solubility?
Molar solubility is the maximum concentration of a solute that can be dissolved in a solvent at a given temperature and pressure.
What factors affect molar solubility?
Molar solubility is affected by molecular weight, polarity, temperature, solvent effects, pH, and common ion effects.
How does temperature affect molar solubility?
Temperature generally increases the molar solubility of solids and decreases the molar solubility of gases.
How does solvent polarity affect molar solubility?
Polar solvents tend to dissolve polar solutes, while nonpolar solvents tend to dissolve nonpolar solutes.
What is the common ion effect?
The common ion effect is the decrease in the solubility of a sparingly soluble salt when a soluble salt containing a common ion is added to the solution.