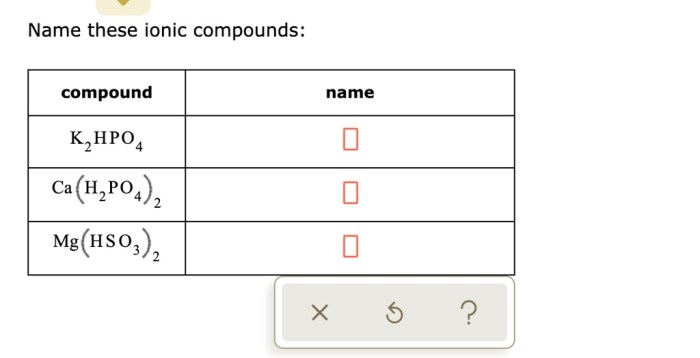
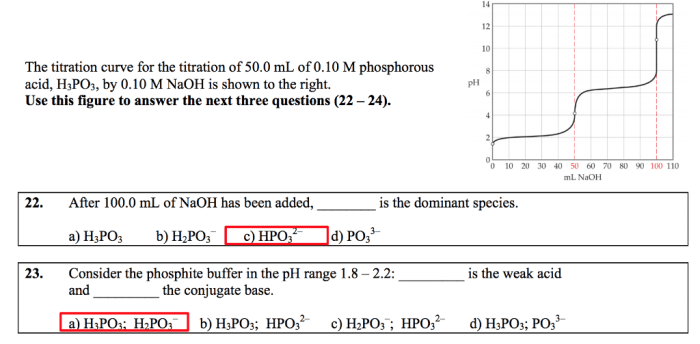
Delving into the intricacies of Ca H2PO3 2 chemical name, this comprehensive guide unveils its significance, properties, sources, applications, and safety considerations. Embark on an enlightening journey that unravels the multifaceted nature of this compound, leaving you with a profound understanding of its role in various scientific and industrial realms.
Ca H2PO3 2, also known as calcium hydrogen phosphite, is a fascinating chemical compound with a unique set of characteristics that sets it apart. This guide will explore its chemical composition, physical and chemical properties, natural occurrence, diverse applications, and essential safety measures to ensure its responsible handling.
Chemical Name and Formula: Ca H2po3 2 Chemical Name
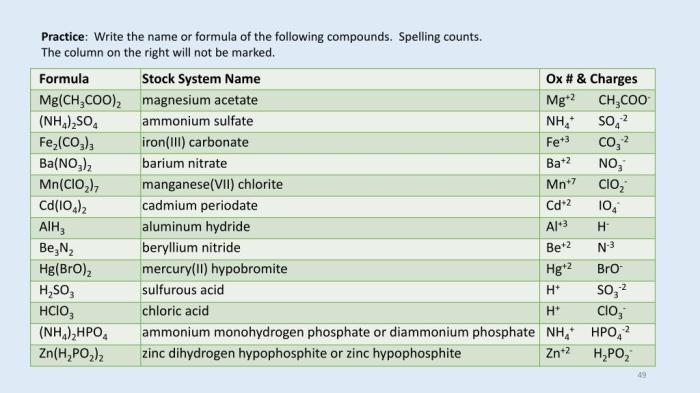
In chemistry, a chemical name is a systematic way of naming chemical compounds. Chemical formulas, on the other hand, use symbols to represent the elements that make up a compound.
The chemical name for “ca h2po3 2” is calcium hydrogen phosphite.
The chemical formula for “ca h2po3 2” is CaH 2PO 3.
The chemical name for CaH2PO3 is calcium dihydrogen phosphite. Calcium dihydrogen phosphite is used in the focusing cup x ray tube to generate X-rays. X-rays are used in a variety of applications, including medical imaging and industrial radiography.
Physical and Chemical Properties
Calcium hydrogen phosphite, also known as calcium dihydrogen phosphite, is a chemical compound with the formula Ca(H 2PO 3) 2. It is a white, crystalline solid that is soluble in water. Calcium hydrogen phosphite is a weak acid that reacts with bases to form salts.
Physical Properties
- Calcium hydrogen phosphite is a white, crystalline solid.
- It is soluble in water.
- It has a density of 2.23 g/cm 3.
- It melts at 190 °C.
- It boils at 400 °C.
Chemical Properties
- Calcium hydrogen phosphite is a weak acid.
- It reacts with bases to form salts.
- It can be oxidized to form calcium phosphate.
- It can be reduced to form calcium phosphite.
Occurrence and Sources
Calcium hydrogen phosphite, or CaH2PO3·2H2O, occurs naturally as a rare mineral called monetite. Monetite is found in phosphate-rich environments, such as guano deposits and marine sediments. It is also a component of some phosphate fertilizers.
Primary Sources
The primary sources of calcium hydrogen phosphite are:-
- Monetite
- Phosphate fertilizers
Applications and Uses
Calcium hydrogen phosphate dihydrate (CaH2PO3·2H2O) finds diverse applications in various industries, primarily due to its unique properties. Its non-toxic nature and versatility make it suitable for a wide range of uses.
Food Industry
- As an anticaking agent, CaH2PO3·2H2O prevents caking and clumping in food products, such as powdered soups, spices, and baking mixes.
- It acts as a stabilizer, maintaining the texture and consistency of processed foods, such as dairy products and sauces.
- In the production of cheese, CaH2PO3·2H2O regulates the acidity level, contributing to the desired flavor and texture.
Agriculture
- CaH2PO3·2H2O serves as a fertilizer, providing essential calcium and phosphorus to plants.
- It helps improve soil structure and fertility, promoting plant growth and crop yield.
- As a dietary supplement for livestock, CaH2PO3·2H2O ensures proper calcium and phosphorus intake for optimal growth and health.
Pharmaceutical Industry, Ca h2po3 2 chemical name
- CaH2PO3·2H2O is used as an antacid, neutralizing stomach acid to relieve indigestion and heartburn.
- In dental applications, it is employed as a polishing agent in toothpaste and as a component in dental cement.
- As a buffer in pharmaceutical formulations, CaH2PO3·2H2O helps maintain the pH of solutions within a specific range.
Other Applications
- CaH2PO3·2H2O finds use in the production of ceramics and glazes, imparting strength and durability to these materials.
- In the leather industry, it is utilized as a tanning agent, enhancing the leather’s quality and durability.
- As a water treatment agent, CaH2PO3·2H2O helps remove impurities and clarify water.
Safety and Handling
Calcium hydrogen phosphite (CaH 2PO 3·2H 2O) requires careful handling to prevent potential hazards. Understanding its properties and following safety guidelines is crucial for ensuring a safe working environment.
General Guidelines
- Wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a dust mask, when handling CaH 2PO 3·2H 2O.
- Avoid direct contact with the skin and eyes, as it can cause irritation.
- Work in a well-ventilated area to minimize inhalation of dust or fumes.
- Store CaH 2PO 3·2H 2O in a cool, dry place away from incompatible materials.
- Dispose of waste properly according to local regulations.
Specific Hazards
- Dust Hazard:CaH 2PO 3·2H 2O can release irritating dust particles when handled or stored improperly. Inhalation of dust can cause respiratory irritation and coughing.
- Eye Irritant:Contact with the eyes can cause redness, pain, and inflammation.
- Skin Irritant:Prolonged or repeated skin contact can lead to dryness, redness, and itching.
- Incompatibility:CaH 2PO 3·2H 2O should not be mixed with strong acids or oxidizing agents, as it can react violently.
FAQ Explained
What is the chemical formula for Ca H2PO3 2?
CaH2PO3
What are the physical properties of Ca H2PO3 2?
Colorless crystals, soluble in water
What are the chemical properties of Ca H2PO3 2?
Acidic, reacts with bases to form salts
What are the applications of Ca H2PO3 2?
Fertilizer, food additive, water treatment
What are the safety considerations for handling Ca H2PO3 2?
Avoid contact with skin and eyes, wear protective gear